Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 ,
, -di(2-ethylhexyl)octanamide from nitric acid medium
-di(2-ethylhexyl)octanamide from nitric acid mediumTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 35(6), p.439 - 449, 2017/08
Times Cited Count:5 Percentile:16.24(Chemistry, Multidisciplinary)Solvent extraction of uranium from a nitric acid medium was performed with  ,
, -di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as
-di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as 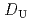 = 1.1
= 1.1![$$[rm NO^{-}_{3}]^{1.6}_{rm aq}[{rm DEHOA}]^{2}_{rm org}$$](/search/images/ERNVY2TNEBHE4XT1FV4V44ZTPVOV24ZRFY1H0X11LRZG0IDBOF4VW402OJWSARCFJBHUC5K3LZ3TE5K5PNOHE1JAN3ZGO5JE.gif) . Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation
. Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation 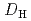 = 0.12
= 0.12![$$[rm H^{+}]^{0.76}_{rm aq}[{rm DEHOA_{rm Free}}]_{rm H}$$](/search/images/ERNVY2TNEBEF24ZLPVOV24ZQFY1TM5K5PNOHE1JAMFYX0W11LRZG0ICEIVEE4QK5PNOHE1JAIZZGKZL3PVOV4402OJWSASD3EQ.gif) was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm
was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm (M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
(M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
 -dialkylamides using multistage mixer-settler extractors
-dialkylamides using multistage mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Tsubata, Yasuhiro; Matsumura, Tatsuro
Procedia Chemistry, 21, p.156 - 161, 2016/12
Times Cited Count:5 Percentile:94.32(Chemistry, Inorganic & Nuclear)A continuous counter-current experiment was carried out to demonstrate the validity of a process using  -dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed
-dialkylamides for recovering U and Pu. This process consisted of two cycles, and the 1st cycle and the 2nd cycle employed  -di(2-ethylhexyl)-2,2-dimethylpropanamide and
-di(2-ethylhexyl)-2,2-dimethylpropanamide and  -di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm
-di(2-ethylhexyl)butanamide as extractants, respectively. The feed solution for the 1st cycle was 5.1 mol/dm (M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5
(M) nitric acid containing 0.92 M U, 1.6 mM Pu, and 0.6 mM Np. The raffinate collected in the 1st cycle was used as the feed for the 2nd cycle. The ratios of U recovered in the U fraction and U-Pu fraction were 99.1% and 0.8%, respectively. The ratio of Pu recovered in the U-Pu fraction was 99.7%. The concentration ratio of U with respect to Pu in the U-Pu fraction was 9, and this indicated that Pu was not isolated. The decontamination factor of U with respect to Pu in the U fraction was obtained as 4.5 10
10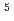 . These results supported the validity of the proposed process.
. These results supported the validity of the proposed process.
 -dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractors
-dialkylamides (DEHDMPA and DEHBA) in mixer-settler extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 34(1), p.37 - 47, 2016/01
Times Cited Count:9 Percentile:29.47(Chemistry, Multidisciplinary)The extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was
-di(2-ethylhexyl)butanamide (DEHBA) for Np(V) and Np(VI) were studied by a batch method using various nitrate ion concentrations. The distribution ratios of Np(VI) obtained with DEHDMPA and DEHBA exceeded unity when the nitrate ion concentration was  3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
3 mol/L. DEHDMPA and DEHBA barely extracted Np(V), and the maximum distribution ratios were 0.4 and 0.2 when DEHDMPA and DEHBA were used as extractants, respectively. A continuous counter-current experiment was performed to evaluate the behavior of Np in a process comprising two cycles. The ratio of Np recovered to the U fraction and U-Pu fraction were 63.7% and 29.1%, respectively. The behavior of Np suggested that the valence state of Np changed from Np(V) to Np(IV) or Np(VI) after the 1st experimental cycle. The recoveries of U and Pu to the U fraction stream and the U-Pu fraction stream were 99.9% and 99.8%, respectively.
 -dialkylamides as extractants for U and Pu by continuous counter-current extractors
-dialkylamides as extractants for U and Pu by continuous counter-current extractorsBan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1147 - 1152, 2015/09
Extraction properties of  -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA),  -di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.
-di(2-ethylhexyl)butanamide (DEHBA), and some of their degradation products for the metal elements Zr, Mo, Ru, Rh, and Pd were studied using a single-stage batch method, and the results showed that the degradation products barely extracted these metal elements. Furthermore, separation performance of DEHDMPA and DEHBA for U and Pu in a continuous counter-current process was evaluated using a calculation code, and it was confirmed that the calculated values of U concentration in the U fraction and U and Pu concentrations in the U-Pu fraction were similar to those measured experimentally. These results supported the applicability of DEHDMPA and DEHBA as extractants for separation processes and the validity of the calculation code for estimating the separation performance of the process.